Enamel Caries Treatment
Machine translation
Original article is written in RU language (link to read it) .
Enamel caries manifests clinically as white or light brown spots. Such a spot is the result of progressive long-term demineralization of the enamel, however, this spot can disappear as a result of establishing the flow of certain mineral components or medicinal drugs into the demineralization focus from the oral fluid.
About the possibilities of remineralization of carious dentin in the webinar "Biomimetic restoration of heavily damaged chewing teeth" Biomimetic restoration of heavily damaged chewing teeth.
The described process of the spot's reverse development has been named "remineralization".
Laboratory and clinically confirmed is the ability of tooth tissues to recover in the initial forms of caries, this ability is due to the main mineral component of enamel – hydroxyapatite crystal, which is capable of modifying its own chemical structure.
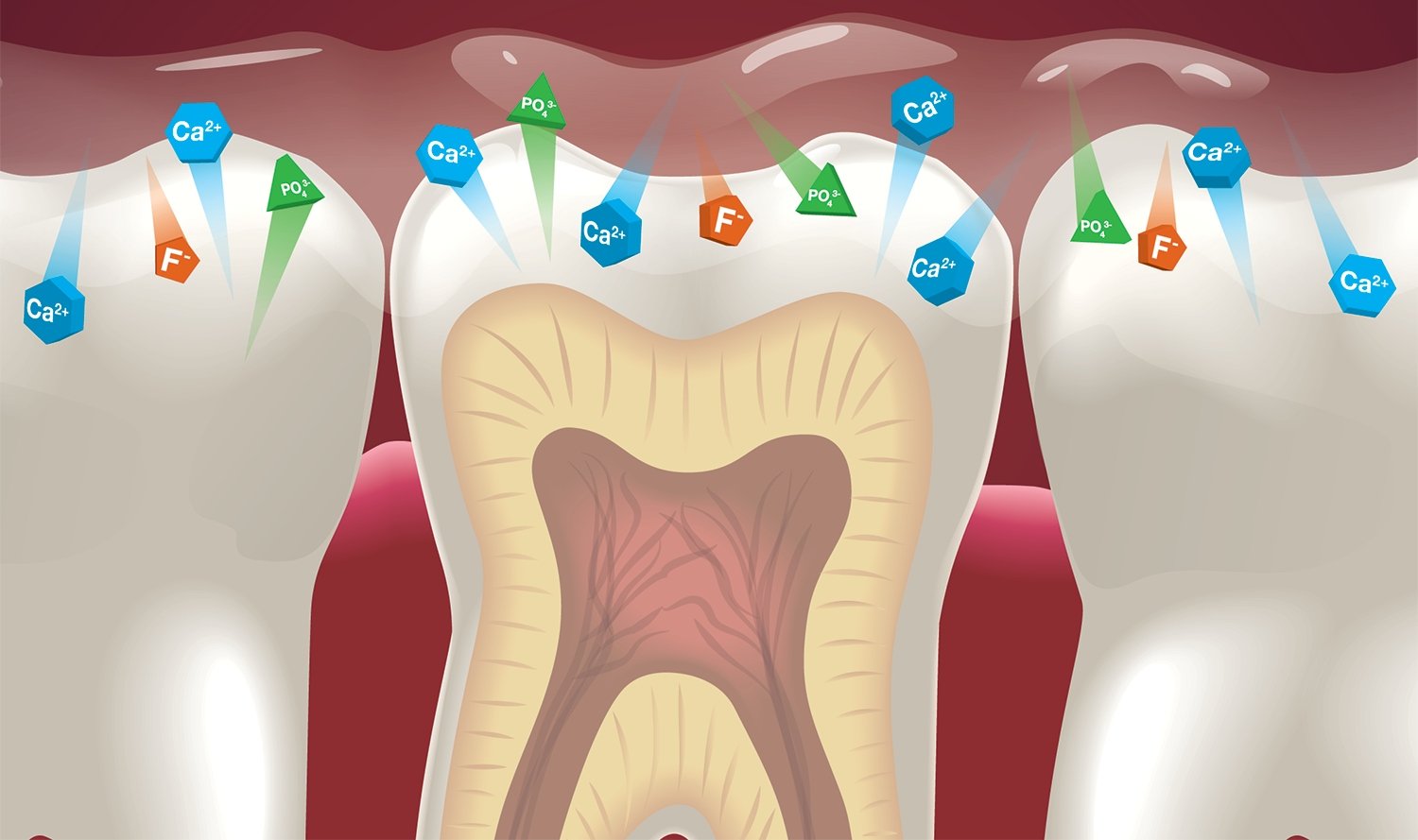
Figure 1. Ion exchange on the surface of the tooth.
In case of loss of a certain portion of calcium or phosphorus ions, under favorable circumstances, hydroxyapatite can regain these lost microelements from saliva through adsorption and diffusion processes, thereby returning to its original state. Hydroxyapatite crystals can also re-form from the phosphate ions and calcium adsorbed by the tooth tissues.
The possibility of remineralization is limited by the degree of involvement of the tooth tissues in the pathological process. The limit of disruption depends on the degree of preservation of the protein matrix. Assuming the preservation of the overwhelming mass of the protein matrix, considering its inherent qualities, it can form bonds with phosphate ions and calcium. Over time, hydroxyapatite crystals will form on it.
In the case of the appearance of a white spot, initial caries, where partial leaching of the mineral components of the enamel (the process of demineralization) is observed, free microspaces remain, but the protein matrix also remains, possessing the ability to remineralize. The high permeability of the enamel in the area of the white demineralized spot facilitates the penetration into this zone of phosphate ions, calcium, fluorides from oral fluid or special remineralizing solutions, forming new hydroxyapatite crystals in place of the lost ones, sealing the microspaces
of the carious spot.
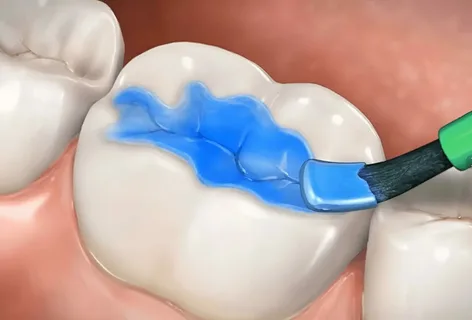
Figure 2. Coating the tooth with a remineralizing compound.
It is important to note that the permeability of different areas of enamel varies, it is not uniform, due to the heterogeneity of its structure. The highest permeability is observed in the following areas of enamel:
- cervical area,
- pits and fissures,
- enamel defects.
The surface layers have the least permeability, while the middle layers of enamel are significantly more permeable. Temperature and concentration of the remineralizing solution, as well as the ability of hydroxyapatite crystals to perform adsorption and ionic exchange of other substances, greatly affect the permeability.
The incorporation of substances into enamel is divided into the following stages:
- ions move from the solution into the hydrated layer of the crystal;
- then ions proceed to the surface of the hydroxyapatite crystal;
- from the surface of the crystal, ions are distributed to different levels of the crystal lattice, and intracrystalline exchange occurs.
The duration of the first stage takes minutes, while the third stage can continue for several tens of days.
Figure 3. Fluoride prevention.
Formations on the tooth surface: pellicle, dental plaque, soft dental deposits, significantly hinder the penetration of most macro- and microelements into the enamel, and obstruct the mechanisms of enamel remineralization. Therefore, all patients must undergo professional hygiene before starting the application of remineralizing agents, clean the tooth surfaces from dental deposits, perform grinding and polishing of teeth, restorations, orthopedic constructions using brushes with abrasive pastes, rubbers, strips. As a quality control, a tongue test can be used – the patient's sensation of smoothness when running the tongue over the tooth surface.
The dentist evaluates the quality of professional hygiene using an angled probe, cotton roll, which should easily glide over the tooth crowns.
The homeostasis of tooth tissues is maintained by the dynamic equilibrium of de- and remineralization mechanisms. A shift in this balance towards the predominance of the demineralization process, slowing down the intensity of remineralization mechanisms, is assessed as a key link in the pathogenesis of caries.
It has been proven that fluoride ions, when in direct contact with the enamel surface, facilitate the restoration of its structure. During the period of amelogenesis and after the tooth erupts, fluorapatite forms in the surface layers of the enamel, which is resistant to the aggressive factors of the oral cavity. Fluoride accelerates the strengthening of calcium in the enamel
by forming fluorapatite, which is characterized by high stability.
Remineralizing therapy can be carried out in various ways, and the result of this process is the reconstruction of the surface layer of damaged enamel.
Remineralization Products
Today, a number of products have been developed that contain calcium ions, phosphate ions, and fluoride, which provide enamel remineralization. The most common products are:
- 10% calcium gluconate solution,
- fluoride-containing gels and varnishes,
- 2% sodium fluoride solution.
Below is a description of a popular tooth enamel remineralization technique.
Procedure Steps
- Using a brush and paste, the tooth surfaces are mechanically cleaned of dental deposits.
- Next, the teeth are treated with a 1% hydrogen peroxide solution and dried with an air stream.
- A segment of altered enamel is covered for 20 minutes with a cotton swab soaked in a 10% solution of calcium gluconate, changing the swabs every five minutes.
- Next comes the application of a 2% sodium fluoride solution, which lasts for five minutes.
At the end of the procedure, the patient is asked to refrain from eating for several hours. The course of remineralization therapy involves 15-20 such daily applications.
Figure 4. Applying the product with a brush.
The effectiveness of the treatment is assessed by the reduction or complete disappearance of the demineralization focus. A more objective method is the staining of the tooth surface where the demineralization focus was previously located with a solution of methylene blue dye. As the structure of the surface layer of the previously damaged enamel is restored, the intensity of its staining will decrease.
The treatment course ends with the application of fluoride varnish on the thoroughly dried crowns of the teeth with a brush.
Results
The white spot as a result of the described treatment method may completely
disappear, and the enamel will restore its natural shine. The possibility of remineralization of the focus is determined by the depth of the lesion in the area of the pathological process. In the case of initial damage, the effect of the therapy is immediately noticeable. If the changes in the enamel were more pronounced, which is clinically manifested by a significant area of damage, and histologically by a disruption of the organic matrix structure, then achieving complete remineralization will not be possible.
There is another remineralization technique – this involves applications of 1-2% sodium fluoride gel on 3% agar.
Procedure Steps
- Performing professional hygiene.
- The heated gel is applied with a brush onto the previously dried tooth surfaces, and it solidifies within a few minutes.
- The treatment course consists of seven applications.
The effectiveness of this technique is significant. By the end of the week-long course, the spot decreases in size several times. A year later, the spots may slightly increase again, but after undergoing another course of therapy, they decrease by another 4-5 times relative to the original condition.
Remineralization using calcium phosphate-containing gel
This is another method to eliminate initial caries. The gel, based on sodium hydrophosphate and calcium chloride, is designed for the reversal of initial caries. Exceptions are advanced cases – these are large spots with impaired permeability, at the center of which a softening focus is identified. Such a spot in the center is preliminarily treated with an acidic gel (pH=5.5). The acidic environment eliminates the damaged tissues in the center of the spot, which are incapable of undergoing remineralization. The rest of the spot is capable of mineralization under the action of the gel's mineral elements, and it gradually recovers.
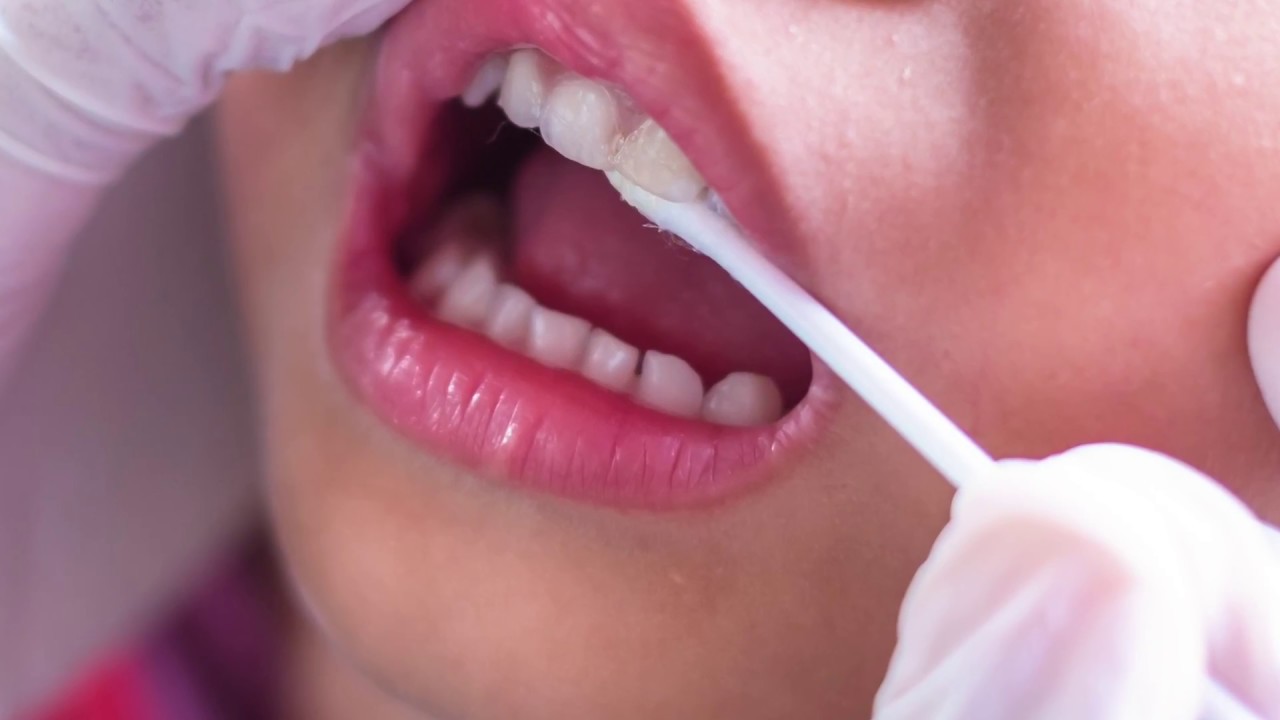
Figure 5. Enamel remineralization in children.
This gel includes calcium ions and phosphate ions in a ratio corresponding to the amount of these elements in saliva (1:4). However, the content of calcium and phosphates in the gel is hundreds of times greater than their amount in saliva. The state of the gel prevents the interaction of phosphates and calcium, which would have led to precipitation.
Treatment Methodology
- Performing professional hygiene.
- Teeth are treated with 0.5% hydrogen peroxide solution, then dried with an air jet.
- Using a brush, all crown surfaces are covered with gel, and dried for several minutes.
The treatment course involves conducting about ten procedures.
For more current information on this topic, visit the webinar Caries and non-caries lesions of milk teeth: from remineralizing therapy to crown placement.
